Our Technology
AMPLIFY Vaccine Platform
HDT Bio’s AMPLIFY vaccine platform combines repRNA coding for an antigen target and LION™, a nanotechnology-based formulation technology that provides a versatile solution for a range of pathologies, from infectious diseases to cancer. The platform offers increased safety and potency, simplified manufacturing, and improved temperature stability compared to traditional RNA vaccines, allowing for increased efficacy with the potential for multivalency.
AMPLIFY compared to current mRNA vaccines
AMPLIFY
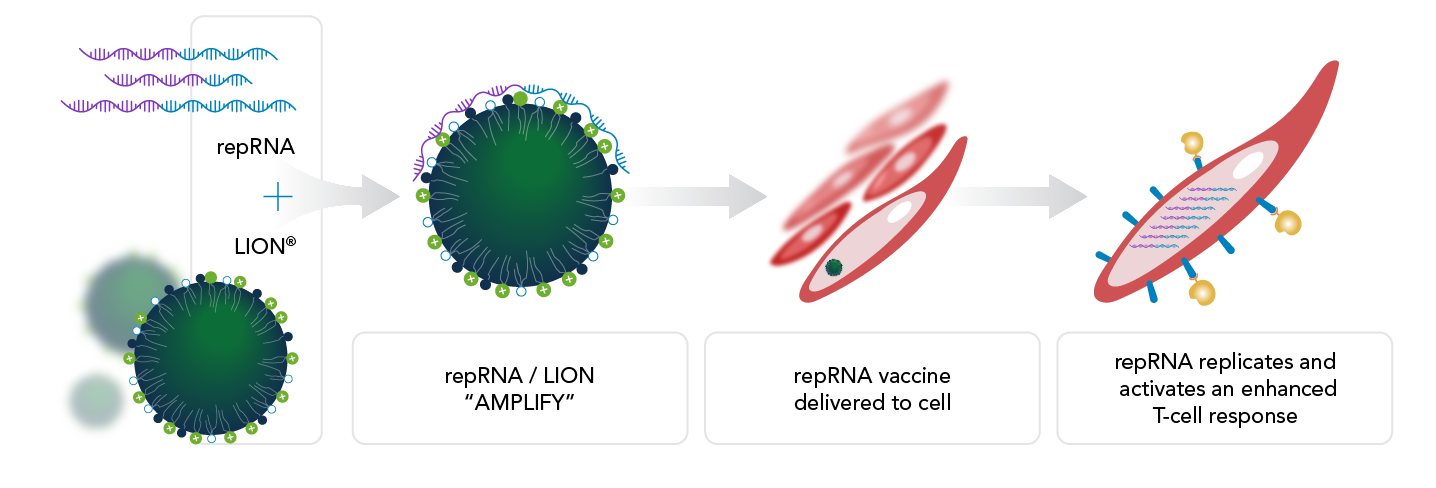
repRNA — Before commencing the translation of antigens, the process involves self-replication within the cell, wherein numerous copies of replicating RNA are generated. This amplification step serves to increase both antigen expression levels and overall potency.
LION — Nanoparticle-based delivery system for independent stockpiling. RNA integration occurs through a simple mixing process, and the RNA attaches to the external surface of the particle, providing stabilization and protection.
Stability — Designed for refrigeration temperatures, not only maintains stability during storage but also guards against RNA degradation, contributing to the overall reliability of the system.
LION maintains a superior safety profile at high doses by stimulating faster cellular uptake compared to LNPs. This approach prevents the viral load from migrating to vital organs, reducing the chances for reactogenicity and serious adverse events.
Current mRNA vaccines
mRNA — Antigen expressed only from each individual strand that arrives in the cell
LNP — Must be co-formulated for encapsulation inside the LNP thereby reducing yield and increasing manufacturing complexity
Stability — Distributable only at hyper-cold temperatures
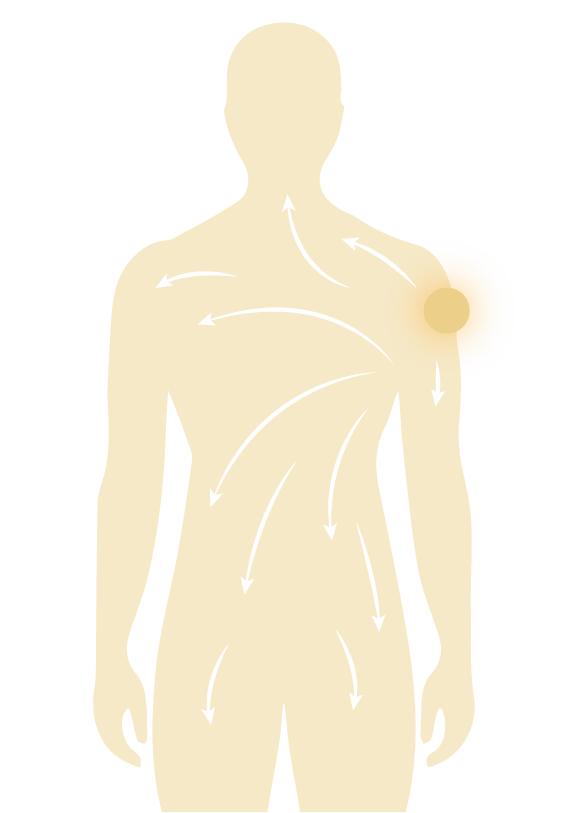
After mRNA/LNP is injected into the body, it is distributed systemically. This distribution induces innate immune reactogenicity which may lead to common or serious adverse events.
Advantages

Superior Safety
Preclinical and clinical studies have demonstrated a favorable safety profile for HDT Bio’s AMPLIFY vaccine platform. Its advanced design and manufacturing processes prioritize rigorous quality control measures, minimizing risks and enhancing patient well-being.

Increased Efficacy
The unique features of LION™ enable precise delivery and robust cellular immune responses, resulting in vaccines that effectively immunize individuals and prevent diseases.
Durable immunogenicity
With a focus on advancing vaccine longevity and durability of immune response, HDT Bio leverages the power of self-amplifying repRNA technology with the LION™ delivery platform to provide individuals with durable protection against targeted diseases.

Improved Stability
Simplified Manufacturing

Enhanced Temperature Stability and Shelf Life
Stability is a hallmark of LION™, a unique vaccine delivery platform that can be rapidly manufactured, lyophilized, and stored at refrigerator temperatures. This feature promotes longer shelf-life, while maintaining potency and effectiveness over time. With LION™, HDT Bio is committed to advancing global health by fighting infectious diseases and improving access to preventative healthcare for all.
Cost-Effective Global Distribution
This technology enables the production and distribution of life-saving vaccines to people all around the world, even in remote and resource-limited areas. The LION™ platform holds the potential to redefine vaccine manufacturing and provide cost-effective solutions for making RNA therapeutics accessible in regions with lower incomes. By prioritizing affordability and accessibility, HDT Bio aims to bridge the gap and ensure that life-saving treatments are available to those who need them most.
ACTIVATE — RIG-I and TLR3 Pathways with RNA Agonists
Activating RIG-I and TLR3 against Cold Tumors
Although immune checkpoint inhibitors have had success in a subset of patients by enabling the immune system to attack cancer, other patients are non-responsive to such drugs and experience ‘cold’ tumors. RIG-I and TLR3 are proteins expressed by most human cells and are responsible for detecting the presence of viruses and initiating inflammatory and immune stimulating responses. These receptors can be activated by appropriately constructed RNAs.
Activation of RIG-I and TLR3 in infection and cancer not only leads to expression of pro-inflammatory cytokines and cancer cell apoptosis, but also induces an innate immune response against the infection or cancer allowing the adaptive immune system to destroy infected cells and metastases and remain vigilant against recurrence. The combination of pathogen associated molecular pattern (PAMP), RNA activating RIG-I and TLR3 and delivery technologies represents a breakthrough in the infectious disease and immuno-oncology fields.
RIG-I Pathway Research from Professor Michael Gale, Jr., and the University of Washington
Professor Michael Gale, Jr., at the University of Washington, is a co-discoverer of the RIG-I pathway and its broad actions in innate immunity and immune programming. He is an expert in infectious disease and innate immunity and is Director of the UW Center for Innate Immunity and Immune Disease. He is a HDT Bio founding scientist and scientific advisor.
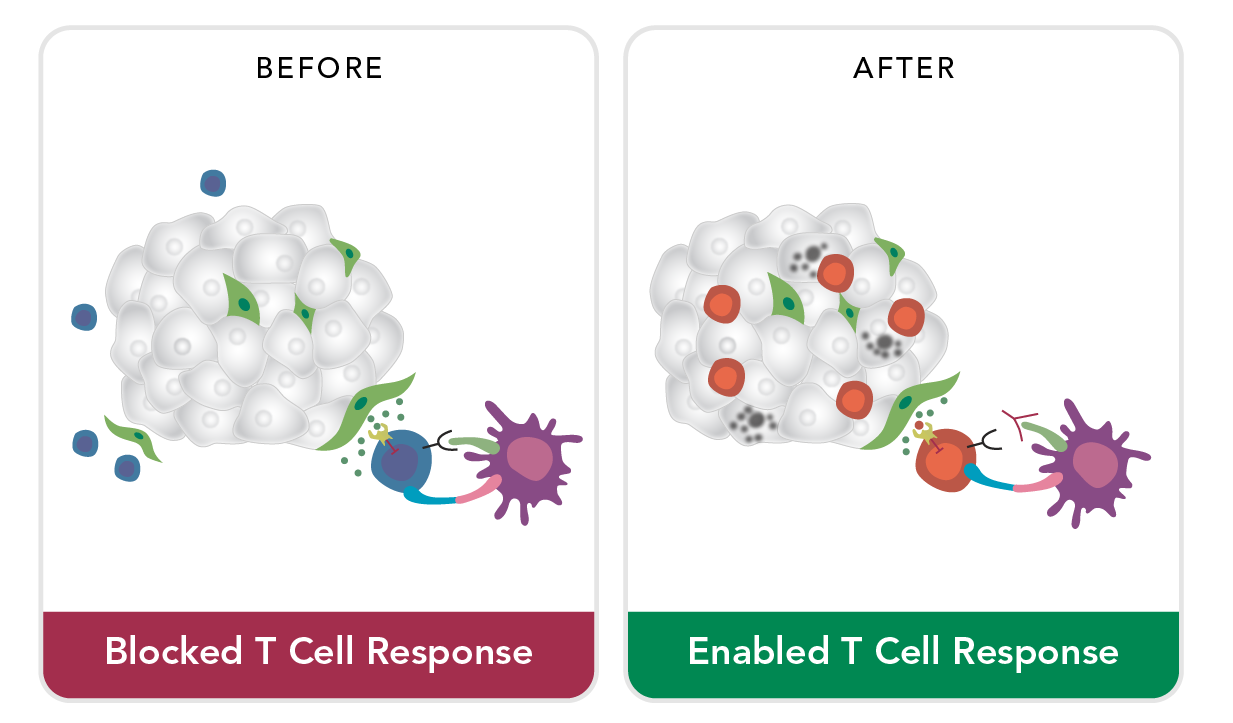
ACCESS via Junction Openers
Viral Protein Research from Professor Andre Lieber and the University of Washington
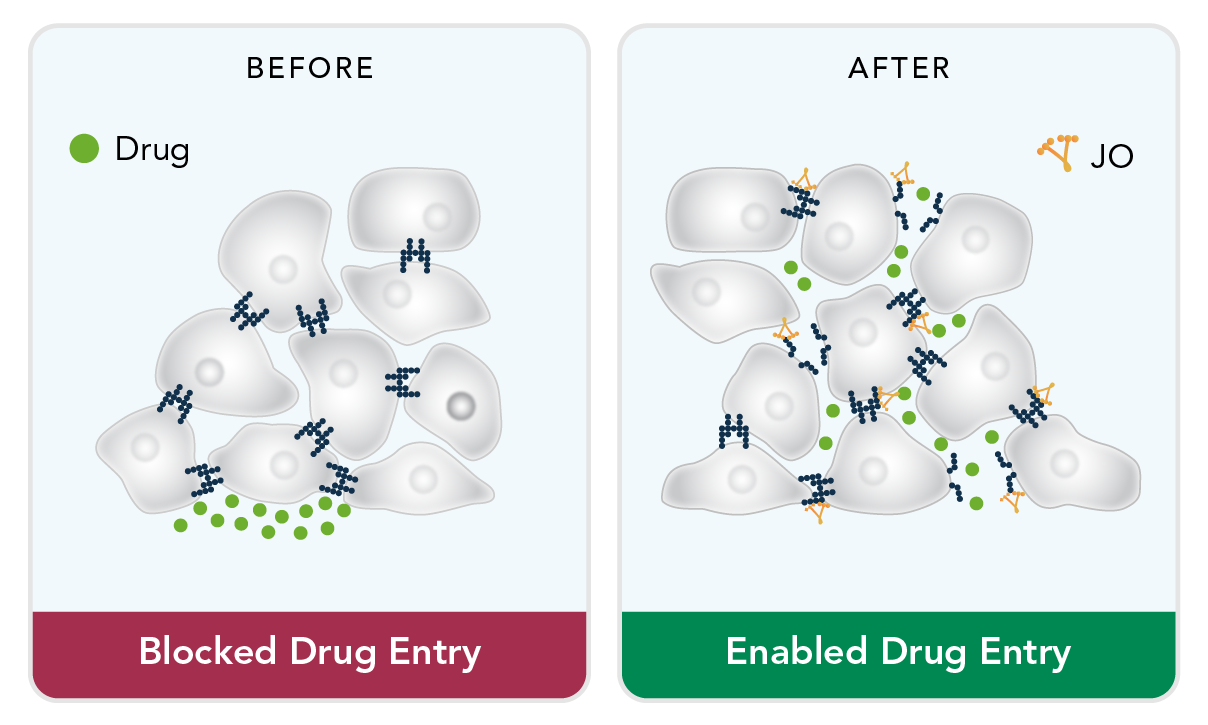
Professor Andre Lieber and his research team at the University of Washington demonstrated that a virus-derived protein can disrupt cellular junctions in epithelial tissue and that such disruption dramatically increases accessibility to the tumor microenvironment. Professor Lieber is a Scientific Advisor to HDT Bio and continues to publish research showing that engineered versions of the protein can increase affinity for tumor junctions and improve the disruptive capability.
Junction Openers for Tumor Penetrance
These so called ‘junction openers’ (JOs) increase the tumor penetrance, and therefore improve the efficacy, of diverse therapeutics including chemotherapeutics (Paclitaxel, Doxil, Cisplatin), antibodies (Herceptin), oncolytic viruses (Adenovirus), and cell therapies (unpublished).
JO variants have undergone extensive preclinical characterization from bioprocess development through immunogenicity testing. Research has demonstrated that monotherapy with JO appears can help the immune system attack a tumor. HDT Bio seeks to use this approach for currently approved cancer treatments and epithelial tumors which account for about 80% of cancers.